BioBall®
Solution for quantitative microbiological quality control
- Calibrated microbial reference
- No preparation time
- Easy to use : BioBall dissolves instantly
További tájékoztatást szeretne?
BioBall® is a small water soluble ball containing a precise number of microorganisms. Produced to the world’s highest quality standards and ISO Guide 34 accredited, it delivers unprecedented accuracy for Quantitative Microbiological Quality Control.
Delivering Confidence in Quantitative Microbiology
Pharmaceutical companies and laboratories are required to test and assure product safety and quality. With BioBall®, discover a calibrated microbial reference that requires no preparation or pre-incubation. BioBall® can be directly added to media or matrix samples, and can be used for many applications :
- Culture media quality control
- Positive control for quantitative Quality Control
- Traditional and alternative methods validation
- Accreditation compliance
- Multi-laboratory standardization
- Determining uncertainty of measurement
- Proficiency schemes
- Antimicrobial effectiveness testing
To find all the available strains, visit the Technical Details tab.
BioBall® : simpler and more precise quantitative microbiology
All our solutions demonstrate high quality and considerable advantages :
- Little or no preparation time : a minimum number of steps
- Easy to use : BioBall® dissolves instantly
- Greater precisions with a known number of cfu
- Consistent number of cfu batch to batch
- Up to 24-month shelf life when stored frozen
Reduces risk of "Failed QC" due to robustness of ALL strains
Products manufactured for your specific microbiology needs
The BioBall® range offers a variety of products and services to meet your needs in terms of:
- Growth Promotion Testing and Validation
- Sterility Assurance Testing
- Antimicrobial Effectiveness Testing
BioBall® Reference Strain
BioBall® SingleShot : Designed for a single inoculation with unprecedented precision, each BioBall SingleShot has a batch mean of between 28 and 33 cfu
BioBall® MultiShot 550 : With a mean of between 500 and 600 cfu , BioBall Multishot 550 is designed to provide 10 precise inoculations (10 x 100µL of 50 cfu) over an 8 hour period : ideal in the microbiology Quality Control where multiple positive controls in the 10 to 100 cfu are needed
BioBall® High Dose 10K : Designed for a single precise inoculation at a higher level of between 8000 and 12000 cfu
BioBall® MultiShot 10E8 : Designed for re-hydration into 1.1 mL of BioBall Re-Hydration Fluid to provide 10 x 100μL doses of between 0.7 and 1.5 x 10E8 cfu, BioBall MultiShot is ideal to test antimicrobial preservation efficacy
BioBall® Mixed Kits : Designed to provide more convenience and flexibility with kits containing the most relevant pharmacopoeia strains.
To find out the available configurations for BioBall Mixed Kits, visit the “Technical Details” tab
BioBall® Custom Services
New service enabling you to order BioBall with the strain that you require :
- From your own environment
- From specified culture collections (preferably DSMZ or NCTC) at any cfu level required
For more information on BioBall Custom Services, click on the “Options & Accessories” tab.
In response to the market trend and to complement our BioBall® Reference Strain offer, we can adapt our offer to your very own specific needs with our BioBall® Custom Services.
This service enables you to order BioBall® with a specific strain : any strains from your in-house collection can be used to manufacture BioBall® products:
- Environmental monitoring isolates
- Contaminants of products
- Raw materials
Available in two different formats (Single Dose with 60 cfu/BioBall or Multi Dose with 550 cfu/BioBall®), it will take approximately 12 weeks for a BioBall Plant Isolate to be manufactured, from the strain reception to its shipment to you.
BioBall® can also be manufactured with any strain selected from specified culture collections (NCTC or DSMZ and can also be made using any proprietary strain with commercial value, as long as third party intellectual property rights are not infringed.
Advantages of a customized service like BioBall® Custom Services
- No upfront cost and free of charge if the specification cannot be met
- You choose the cfu level most adapted to your needs : more flexibility and convenience for your everyday needs
- Real experience in Quantitative microbiology
- Supplied certificate of analysis stating actual mean cfu and standard deviation for each batch
- BioBall® Plant Isolate has a 12-month expiry date
- Worry free ordering : from collecting your strains to delivering BioBall® Plant Isolate to your site – BTF organizes everything
- Second and subsequent orders of the same strains in a BioBall Plant Isolate format are eligible for a discount
Manufacturing BioBall® using flow cytometry
bioMérieux uses patented technology and proprietary techniques to manufacture BioBall® products in Australia.
A flow cytometer is used to select and dispense individual cells within a culture, and count them into a single droplet.
Each droplet therefore contains a precise number of viable cells that have been individually selected for maximum recovery rate on non-selective media.
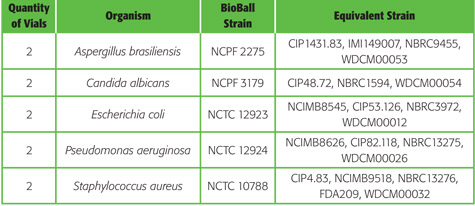
Available strains for BioBall®
Discover the list of strains we can put into a standard BioBall® Format:
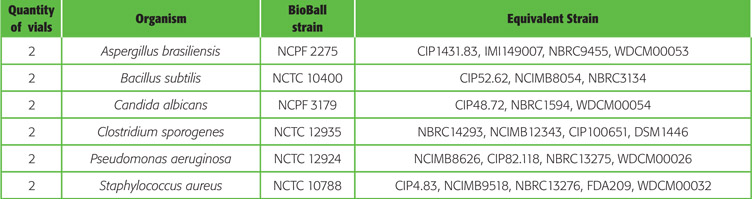
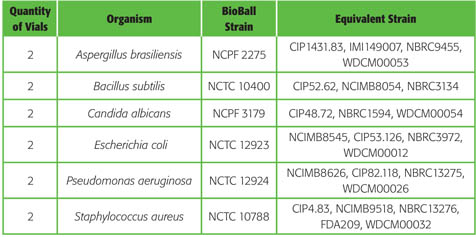
BioBall® Mixed Kits : flexibility in quantitative microbiology
5 configurations of BioBall® Mixed Kits are currently available, containing the most relevant pharmacopoeia strains required for quantitative microbiology.
BioBall SingleShot and BioBall MultiShot 550 mixed kit for Sterility Assurance Testing
btf_bioball_12pp_brochure_table1.jpg
btf_bioball_12pp_brochure_table2.jpg
btf_bioball_12pp_brochure_table3.jpg