VIDAS® TB-IGRA
Reliable and fully automated solution for Tuberculosis (TB) infection detection
Available on the VIDAS® 3 immunoanalyzer, VIDAS® TB-IGRA is a whole-blood test for the diagnosis of Mycobacterium tuberculosis infection.
- No sample preparation
- Low hands-on time
- Fully automated process from sample distribution to result
- Reliable result within 17 hours for one patient
További tájékoztatást szeretne?
TAKE CONTROL OF YOUR TB-IGRA testing with VIDAS®3
Easily improve your workflow and save time - Detect more TB-infected individuals
Adequate diagnosis and treatment of Latent Tuberculosis Infection (LTBI) is one of the critical components of the World Health Organization’s End TB Strategy, essential to prevent the development of Active Tuberculosis disease and stop the spread of Tuberculosis.
Our new TB Interferon-Gamma Release Assay, VIDAS® TB-IGRA, offers a simple, efficient, and reliable solution to perform in-house diagnosis of TB infection.
Optimize your workflow with an easy TB-IGRA testing solution and save time*
ALL STEPS MANAGED INSIDE VIDAS 3 from a single whole blood tube

Gain in efficiency by managing
|
Detect more TB-infected individuals*Provide reliable results for TB infection
|
* Compared with QuantiFERON-TB Gold Plus (QFT-Plus) - QuantiFERON® is a trademark belonging to Cellestis - See PERFORMANCE tab for further details
** Compared to the gold standard: culture
The clinical performance characteristics of the VIDAS® TB-IGRA assay were evaluated on two populations: patients with active TB and persons at mixed risk of Mtb infection.
MORE CONFIDENCE IN IDENTIFYING INFECTED PEOPLE
HIGH SENSITIVITY
ON ACTIVE TB POPULATION
The VIDAS® TB‑IGRA assay gave
- more true‑positive results,
- fewer false‑negative results,
than the comparative assay.
HIGH SPECIFICITY
ON POPULATION AT EXTREMELY LOW LEVEL OF TB INFECTION **
|
High capacity to identify active TB patients |
STRONG AGREEMENT WITH COMPARABLE SOLUTION
ON POPULATION WITH MIXED RISK OF TB INFECTION***
As there is no gold standard for the diagnosis of individuals with Latent TB Infection (LTBI), PPA and NPA with the comparative assay were determined.
| Detect more TB-infected individuals as risk becomes greater |
Positive results of the VIDAS® TB‑IGRA (■)versus QFT®‑Plus (■) in the Mix Risk population

Rate of positive results was statistically higher (α=5%) for the VIDAS® TB‑IGRA assay than for the QFT®‑Plus assay (p‑value < 0.0001).
As the TB exposure risk increases, VIDAS® TB IGRA detects more positive samples reflecting a stronger association with the expected likelihood of Latent TB Infection
REDUCE CHALLENGES LINKED TO INDETERMINATE RESULTS
 VIDAS® TB-IGRA assay provides fewer indeterminate results***,
VIDAS® TB-IGRA assay provides fewer indeterminate results***,
HIGH CAPACITY TO GIVE VALID, INTERPRETED RESULTS TO CLINICIANS
The rate of indeterminate results was statistically lower (α=5%) for the VIDAS® TB‑IGRA assay than for the QFT®‑Plus assay (p‑value = 0.0001).
|
Reliable results for TB infection, Greater ability to detect Mycobacterium tuberculosis infected persons |
** Blood donors from a low endemic country considered at extremely low risk of TB infection
*** Compared to QuantiFERON® Gold Plus test (QFT®-Plus)
Tuberculosis (TB) remains a major global health problem and a leading cause of death worldwide with close to 4000 lives a day. It causes approximately 10 million new cases each year. TB is a communicable disease caused by infection with Mycobacterium tuberculosis. TB is transmitted from person to person, for example when people with pulmonary TB expel bacteria by coughing. With a timely diagnostic and an adapted antibiotic treatment, most people who develop TB disease can be cured.
1.https://www.who.int/teams/global-tuberculosis-programme/tb-reports
2.https://www.ecdc.europa.eu/en/tuberculosis
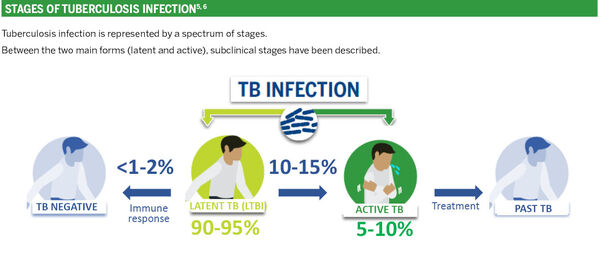
LATENT TUBERCULOSIS, A HUGE RESERVOIR FOR A DEADLY DISEASE
DIAGNOSIS OF LATENT TUBERCULOSIS INFECTION (LTBI)
A priority to:
- Prevent the development of TB disease
- Stop the spread of TB
CE Marked. Other health authorities approvals pending